Introduction
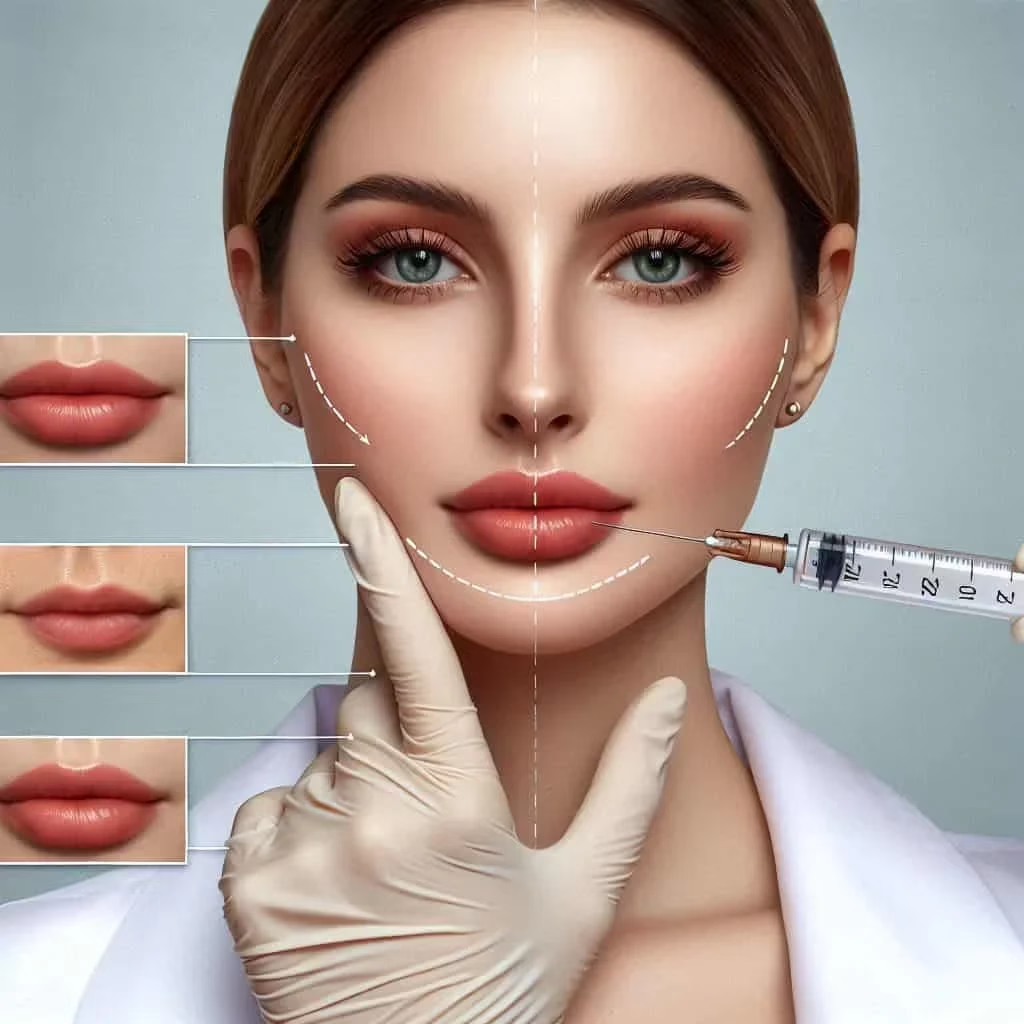
Hyaluronic acid (HA) dermal fillers are a vital tool in aesthetic medicine, providing minimally invasive solutions for addressing facial wrinkles, volume loss, and contour irregularities. While these treatments are generally safe and effective, complications or patient dissatisfaction can necessitate the reversal of fillers. This comprehensive guide explores the use of hyaluronidase in dissolving dermal fillers, detailing dosages, techniques, consent processes, and the critical role of ultrasound in enhancing procedural precision and safety.
Understanding Hyaluronic Acid Fillers
Hyaluronic acid fillers are composed of cross-linked synthetic HA, a naturally occurring biopolymer responsible for hydration and tissue support. The degree of cross-linking in fillers determines their viscosity, longevity, and ease of dissolution, influencing the protocols required for their reversal.
- Highly Cross-Linked Fillers: These provide longer-lasting results but require higher doses of hyaluronidase for effective breakdown.
- Soft or Lightly Cross-Linked Fillers: Easier to dissolve but require precise targeting due to their propensity for diffusion into surrounding tissues.
Practitioners must be familiar with the specific rheological properties of different fillers, including elasticity, cohesivity, and particle size, to effectively plan for both administration and potential reversal.
Mechanism of Action of Hyaluronidase in Dissolving Dermal Filler
Hyaluronidase enzymatically hydrolyses the glycosidic bonds in hyaluronic acid, reducing its molecular weight and viscosity. This process transforms HA fillers from a gel-like consistency into smaller molecules that are readily absorbed by the body.
Key Factors Affecting Hyaluronidase Activity
- Filler Properties: Cross-linking density and particle size impact the rate of enzymatic breakdown.
- Concentration of Hyaluronidase: Higher concentrations expedite dissolution in emergencies, while lower doses are suitable for gradual corrections.
- Individual Variability: Patient-specific factors such as metabolic activity and tissue composition influence the response to hyaluronidase.
Indications for Dissolving Dermal Filler
Hyaluronidase is indicated in a variety of clinical situations:
Aesthetic Concerns
- Asymmetry: Uneven filler distribution, causing facial disharmony.
- Overcorrection: Excessive filler volume leading to an exaggerated appearance.
- Tyndall Effect: A bluish discolouration resulting from superficial filler placement.
- Nodule Formation: Lumps caused by migration or improper integration of filler.
Complications
- Vascular Occlusion: A critical emergency requiring immediate intervention to prevent tissue necrosis or blindness.
- Infection: Used alongside antibiotics to address infections related to filler placement.
- Delayed Hypersensitivity Reactions: Immune-mediated responses causing swelling, redness, or induration.
Patient Preference
Patients may request filler reversal due to dissatisfaction with outcomes, changing aesthetic goals, or a desire to return to a natural appearance.
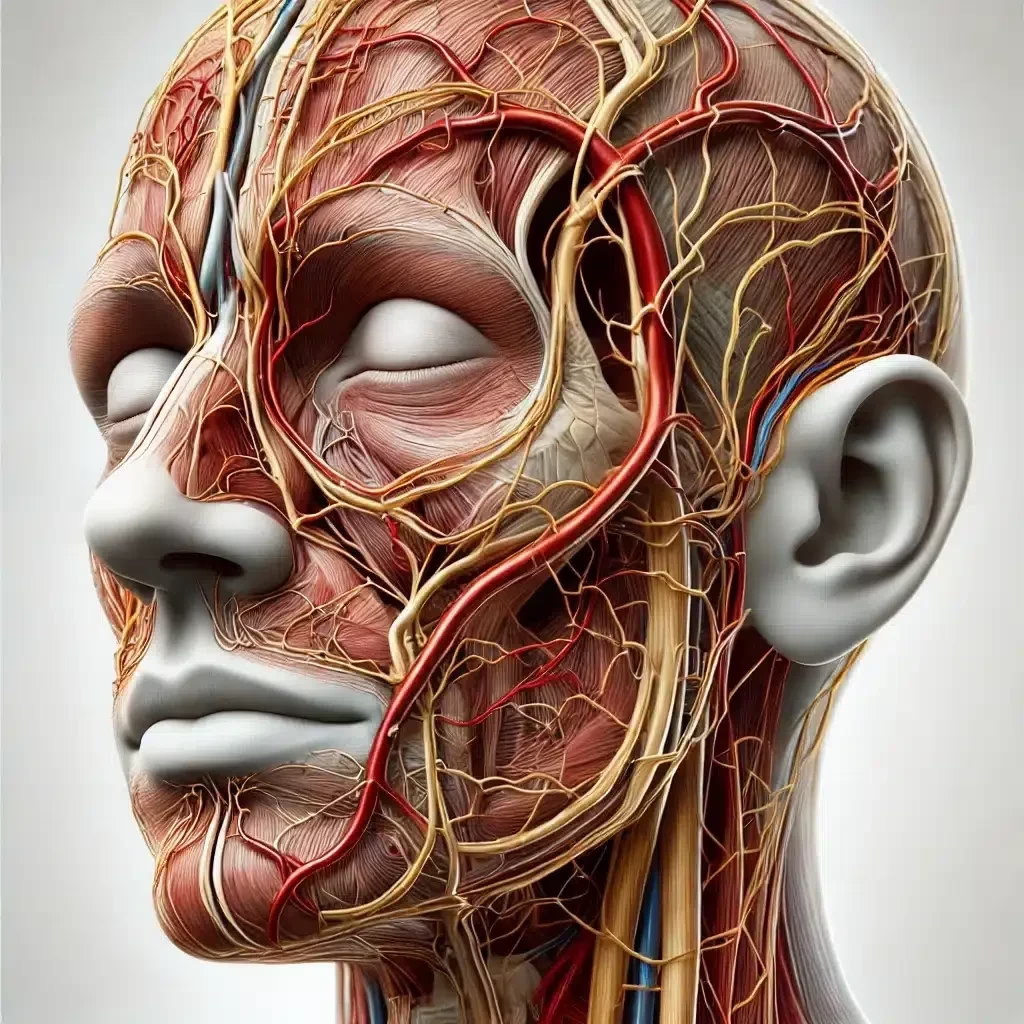
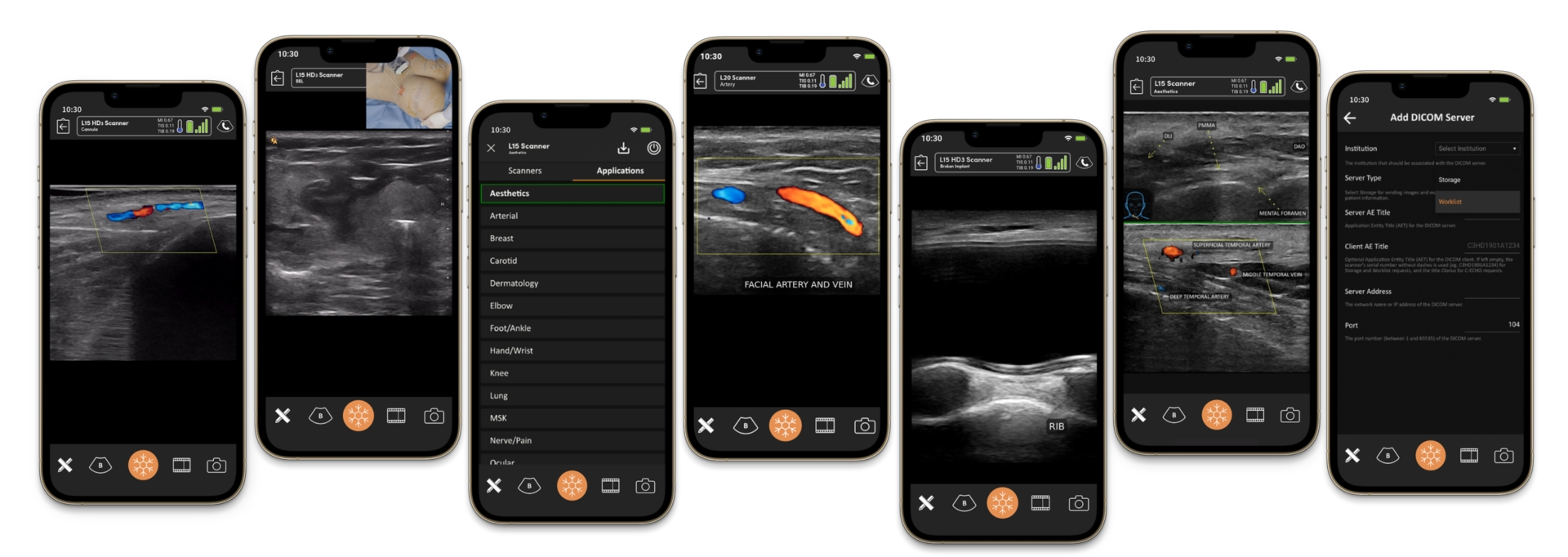
Ultrasound in Diagnosing Vascular Occlusion and Enhancing Hyaluronidase Precision
The advent of high-resolution ultrasound has greatly enhanced the safety and efficacy of filler management, particularly in diagnosing vascular occlusion and guiding hyaluronidase placement.
Diagnostic Benefits of Ultrasound in Dissolving Dermal Filler
- Real-Time Imaging: Provides immediate visualisation of vascular structures and filler deposits.
- Differentiation Between Bruising and Occlusion: Doppler ultrasound can confirm blood flow in suspected occlusions, distinguishing them from benign bruising.
- Precise Mapping of Vascular Anatomy: Facilitates identifying occluded vessels and their relationship to filler placement.
- Early Detection of Hypoxic Changes: Ultrasound can identify tissue oedema or other early signs of ischaemia, allowing prompt intervention.
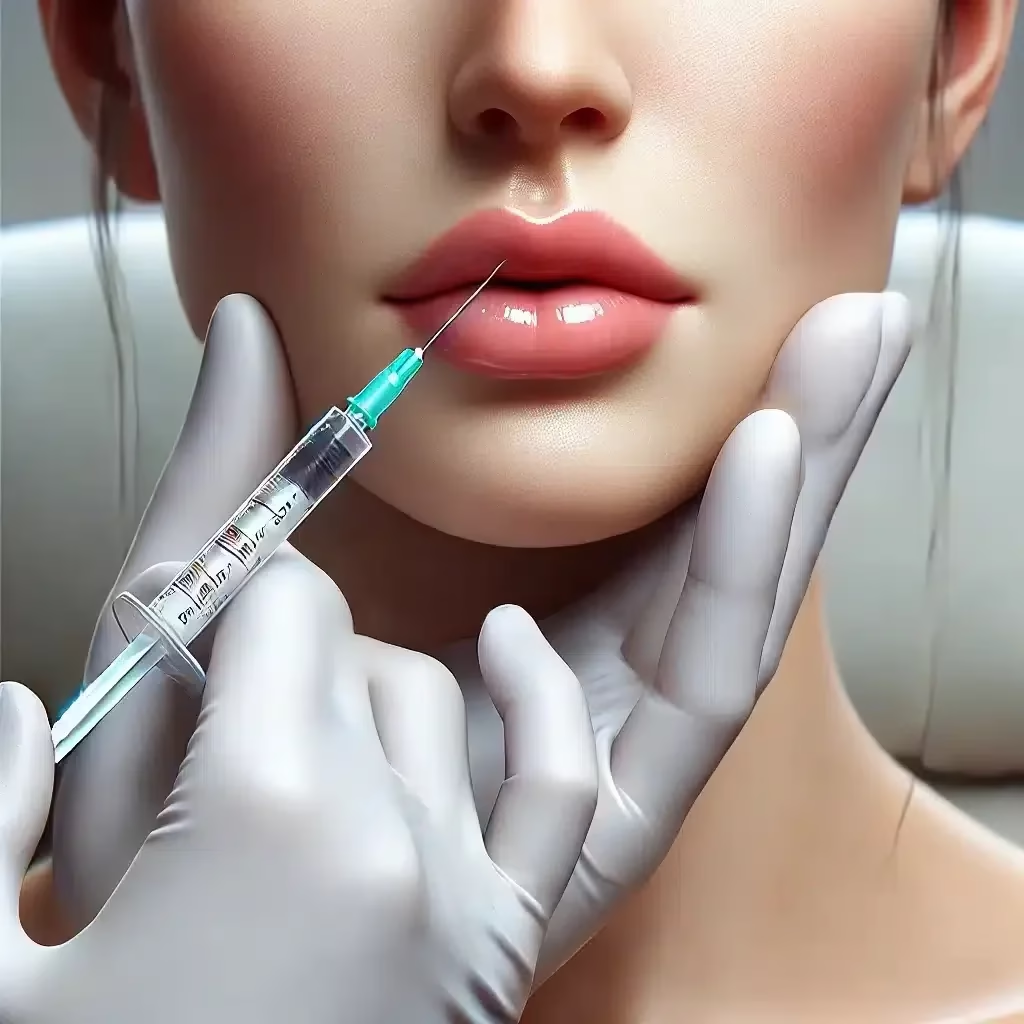
Ultrasound-Guided Hyaluronidase Injections
Ultrasound enables precise localisation and targeted dissolution of filler, minimising the risks of over-dissolution and damage to surrounding tissues.
Procedure Steps:
- Preparation: Use a high-frequency linear transducer (7–15 MHz) with sterile gel for optimal acoustic contact.
- Visualisation: Identify the filler deposit, which appears as a hypoechoic structure, and its proximity to critical structures.
- Needle Guidance: Insert the needle under ultrasound guidance to ensure accurate placement.
- Injection: Administer hyaluronidase in small aliquots (0.05–0.1 ml), monitoring real-time filler breakdown.
- Reassessment: Post-procedure ultrasound ensures the complete dissolution of the filler or identifies the need for additional doses.
Dosage and Dilution Protocols for Hyaluronidase
Proper dosing and dilution are critical for safe and effective filler reversal.
General Dilution Recommendations
- Low Concentration (15 IU/ml): For superficial fillers, gradual dissolution, or delicate areas such as the periorbital region.
- Moderate Concentration (45 IU/ml): Appropriate for mid-depth corrections or aesthetic refinements.
- High Concentration (150 IU/ml): Used in emergencies, such as vascular occlusion, for rapid filler breakdown.
Elective Reversal Doses
- Dose: 30–75 IU per site.
- Dilution: 45–150 IU/ml, targeting specific areas for correction with 0.05–0.1 ml aliquots.
Emergency Reversal Doses
- Initial Dose: 150–300 IU in a 150 IU/ml solution.
- Technique: Serial puncture injections, depositing 0.1–0.2 ml aliquots along the affected vessel.
- Repeat Dosing: Administer additional injections every 15–30 minutes as needed.
Comparing Vascular Occlusion and Bruising
Differentiating between vascular occlusion and bruising is critical for appropriate management.
Key Differences
| Feature | Vascular Occlusion | Bruising |
|---|---|---|
| Cause | Intravascular filler injection | Capillary injury |
| Appearance | Blanching followed by mottling | Purple or blue discoloration |
| Pain | Severe, disproportionate | Mild to moderate |
| Capillary Refill | Delayed or absent | Normal |
| Temperature | Cool or cold | Warm |
| Resolution | Requires intervention | Self-limiting |
When in Doubt
In ambiguous cases, ultrasound and clinical markers should guide decision-making. Administering hyaluronidase in suspected occlusion cases is often justified to mitigate risks of necrosis or blindness.
Consent for Dissolving Dermal Filler
Informed consent is vital, whether for elective or emergency hyaluronidase administration.
Elective Use
- Explanation: Discuss risks, benefits, and potential outcomes, including the need for multiple sessions.
- Documentation: Record the patient’s aesthetic concerns, clinical findings, and agreed-upon plan.
- Photography: Take pre- and post-procedure photographs for transparency and legal documentation.
Emergency Use
- Verbal Consent: In emergencies, verbal consent is sufficient. Provide a concise explanation of the urgency and procedure.
- Post-Treatment Discussion: After stabilisation, thoroughly explain the event and intervention to the patient.
- Detailed Records: Document clinical observations, actions taken, and outcomes.
Post-Treatment Care After Dissolving Dermal Filler
After hyaluronidase administration, patients should:
- Avoid strenuous activity and direct sun exposure for 24 hours.
- Use cold compresses to manage swelling.
- Refrain from applying makeup for 12–24 hours.
- Attend follow-ups to assess tissue recovery and residual filler.
Potential Adverse Effects of Dissolving Dermal Filler
While generally safe, hyaluronidase may cause:
- Bruising and Swelling: Mild and transient.
- Pain or Tenderness: Can be mitigated with local anaesthetics.
- Temporary Softening: Resolves as endogenous HA is replenished.
- Allergic Reactions: Rare but significant; patch testing is recommended in non-urgent cases.
Conclusion
The ability to reverse hyaluronic acid fillers with hyaluronidase is a cornerstone of safety in aesthetic medicine. The integration of ultrasound for diagnosing vascular occlusion and guiding precise enzyme placement has elevated procedural standards, ensuring optimal outcomes and enhanced patient safety. By combining technical expertise, evidence-based protocols, and robust consent processes, practitioners can confidently manage filler complications and aesthetic corrections while maintaining trust and satisfaction among patients.
References
- The Aesthetic Complications Expert (ACE) Group. (2023). Guidelines on Hyaluronidase Use in Aesthetic Practice.
- Kim, Y. J., & Koh, I. S. (2020). New High-Dose Pulsed Hyaluronidase Protocol. Journal of Clinical and Aesthetic Dermatology.
- Jones, D., et al. (2023). Filler Resistance to Hyaluronidase. Aesthetic Surgery Journal.
- DeLorenzi, C. (2014). Complications of Injectable Fillers, Part 2. Aesthetic Surgery Journal.
- BAAPS (British Association of Aesthetic Plastic Surgeons). Guidelines for Emergency Management in Aesthetic Practice.
Disclaimer: This article about Dissolving Dermal fillers is informational and should not replace clinical judgment or professional training.