Introduction to Dissolving Dermal Fillers
The world of aesthetic medicine has been revolutionised by hyaluronic acid (HA) dermal fillers. These injectable treatments offer a minimally invasive way to restore volume, smooth wrinkles, and enhance facial contours, providing patients with immediate, noticeable results. However, even in the most skilled hands, complications can arise, ranging from minor aesthetic imperfections to serious medical emergencies like vascular occlusions. This is where hyaluronidase (Hyal) becomes an indispensable tool for aesthetic practitioners. Often referred to as the “antidote” to HA fillers, hyaluronidase is an enzyme that breaks down hyaluronic acid, effectively reversing the effects of the filler and mitigating any unwanted outcomes.
This comprehensive guide will explore the science behind hyaluronidase, its various applications, proper usage techniques, and crucial considerations for ensuring patient safety. We’ll explore common complications and provide detailed protocols for managing them effectively.
Understanding Hyaluronidase: The Key to Reversing HA Fillers
Hyaluronidase is an enzyme that specifically targets and degrades hyaluronic acid (HA). It works by hydrolysing the glycosidic bonds holding the HA molecules together, breaking them into smaller, more easily absorbed fragments. Hyaluronidase has an immediate effect with a half-life of about 2 minutes, but its clinical effects can last 24-48 hours. The growing experience with Hyal products has resulted in an acceptable safety profile. In non-emergent complications, such as the Tyndall effect, non-inflamed nodules, and allergic reactions, low to moderate doses of Hyal are recommended. In emergency complications, such as vascular occlusion and blindness, immediate, high-dose Hyal treatment is recommended.
Types of Hyaluronidase: A Spectrum of Options
Not all hyaluronidase formulations are created equal. They differ in their source, concentration, and potential for allergic reactions. The three FDA-approved options available in the United States are:
- Bovine-derived (Amphadase): Extracted from bovine (cattle) testes. It’s been around for a while and is generally effective but carries a slightly higher risk of allergic reactions compared to other options.
- Ovine-derived (Vitrase): Derived from ovine (sheep) testes. Similar to bovine-derived hyaluronidase in terms of efficacy, it might also pose a slightly elevated risk of allergic reactions.
- Recombinant Human (Hylenex): Produced through recombinant DNA technology, meaning it’s synthesised in a lab using genetically modified microorganisms. Because it’s not derived from animals, Hylenex is considered to have the lowest risk of allergic reactions, making it a preferred choice for many practitioners.
When choosing a hyaluronidase product, consider the patient’s allergy history and the urgency of the situation. In emergency situations, the potential benefits of using any available hyaluronidase may outweigh the risk of a mild allergic reaction. However, selecting recombinant human hyaluronidase might be a safer approach for non-urgent cases.
Preparation and Storage: Maximising Hyaluronidase Efficacy
Proper handling is crucial for preserving Hyal’s effectiveness. The recommendation is to reconstitute Hyal with bacteriostatic normal saline. Once reconstituted, it should be stored in a cool place, between 2°C to 8°C (35°F to 46°F). It is highly recommended that the product is used immediately after preparation to optimise its efficacy.
Dosage: A Tailored Approach
Determining the appropriate hyaluronidase dosage is a critical step in achieving optimal results. Overzealous use can lead to unwanted hollowing or volume loss, while insufficient doses may not fully resolve the complication. Several factors influence the ideal dosage:
- Type of HA Filler: Different fillers have varying degrees of crosslinking, which affects their resistance to hyaluronidase. Highly cross-linked fillers, often used for structural support, may require higher doses compared to less cross-linked fillers, which are used for fine lines.
- Volume of Filler: The amount of filler to be dissolved directly correlates with the required hyaluronidase dosage. Larger volumes generally necessitate higher doses.
- Location of Filler: Filler in certain areas, such as the lips or tear troughs, may be more sensitive to hyaluronidase, requiring lower doses to avoid over-correction.
- Type of Complication: The nature of the complication influences the dosage. Minor aesthetic issues may require conservative doses, whereas vascular occlusions demand aggressive, high-dose treatment.
- Desired Outcome: Do you want to fully dissolve all of the HA or just soften the area a little? This depends on the complication being treated and the original desired outcome of the filler.
A titration approach is recommended. Start with a conservative dose and gradually increase it until the desired result is achieved. This allows for careful monitoring and prevents over-correction.
Here’s a general dosage guideline:
Table 1: Hyaluronidase Dosage Guidelines for Different Complications
| Complication | Dosage (IU) | Notes |
|---|---|---|
| Tyndall Effect | 10-75 | Follow up to check if more treatment is needed |
| Non-Inflamed Nodules | 5-150 | Dosage depends on how severe the issue is and the filler type |
| Inflammatory Nodules | 500 | Administered every 48 hours and often along with other treatments |
| Vascular Occlusion | 450-1500 | Up to four cycles may be needed. Ultrasound guidance for more accurate results is highly recommended to lower risks. |
These are only guidelines. Clinical expertise is paramount.
Injection Techniques: Optimising Hyaluronidase Delivery
The method of hyaluronidase injection can significantly impact its effectiveness. Two primary techniques are commonly employed:
- Surface Application: Injecting hyaluronidase around the exterior of the filler mass.
- Direct Injection: Injecting hyaluronidase directly into the filler mass to enhance degradation
Studies have shown that direct injection is more effective, particularly for denser, highly crosslinked fillers. Direct injection allows the enzyme to penetrate the filler more thoroughly, accelerating the breakdown process.
Navigating Common Complications: A Step-by-Step Guide
Hyaluronidase is a vital asset in treating a range of filler-related issues. Let’s break down some of the most common scenarios:
- Tyndall Effect: This bluish discolouration occurs when filler is placed too superficially. Hyal can effectively dissolve the misplaced filler, restoring normal skin tone. A small amount of hyaluronidase (e.g., 10-75 units) injected directly into the affected area, followed by gentle massage, can usually resolve the issue.
- Overfilling: Correcting overfilling requires patience and a gradual approach. Start with a low dose of hyaluronidase and assess the results after a few days. Repeat the injections as needed until the desired contour is achieved.
- Non-Inflamed Nodules: These bumps can arise from filler clumping. Hyal helps break down the filler, smoothing the skin’s surface. A small amount of hyaluronidase (5-150 units) injected directly into the nodule can soften and dissolve the filler, restoring a smooth contour.
- Inflammatory Nodules: These may indicate an infection or inflammatory reaction. Hyal is often used in conjunction with antibiotics and/or corticosteroids to resolve the nodule. Larger doses (e.g., 500 units) may be required, and the injections may need to be repeated every 48 hours.
- Vascular Occlusion: This is an emergency. The most common signs of VO are pain, skin colour change, tissue blanching, delayed capillary refill, livedo reticularis, and vision problems. Immediate injection of high-dose Hyal is necessary to restore blood flow and prevent tissue necrosis. Ultrasound guidance can improve the precision and effectiveness of the Hyal injections. A prompt diagnosis is vital. Signs include blanching, pain, and changes in skin colour. High-dose hyaluronidase should be injected immediately, directly into and around the affected area and potentially along the path of the occluded vessel. Frequent reassessment is needed. Adjunctive treatments, such as warm compresses and massage, may also be helpful.
- Vision Loss: This requires immediate intervention. High doses of hyaluronidase should be administered as quickly as possible, and the patient should be referred to an ophthalmologist immediately. The exact protocol for vision loss is still debated, but prompt action is crucial.
The Controversial Topic: Skin Pretesting
Allergic reactions to hyaluronidase are uncommon, but they can occur. Some practitioners advocate for skin pretesting to identify potential allergies before treatment. However, skin pretesting is not required for emergencies. It is optional for non-emergencies, especially when using animal-derived products.
If pretesting is performed, a small amount of hyaluronidase is injected intradermally (just beneath the skin) and observed for any signs of allergic reaction, such as redness, swelling, or itching. A negative skin test doesn’t completely rule out the possibility of an allergic reaction, but it can help to identify individuals at higher risk.
Addressing Key Questions
Let’s address some common questions surrounding hyaluronidase use:
- How long does it take for hyaluronidase to work? Hyaluronidase starts working almost immediately to break down HA. The visible effects can be seen within 24-48 hours.
- Can hyaluronidase damage my own natural hyaluronic acid? Yes, but only in the localised treatment area. Hyaluronidase can break down the body’s natural HA, but the effect is temporary, as the body will replenish it.
- Can hyaluronidase fix asymmetry caused by fillers? Yes. By strategically injecting hyaluronidase, asymmetry can be corrected by dissolving filler in areas where excessive volume is present.
- Does hyaluronidase hurt? Injection of hyaluronidase can cause some discomfort, similar to filler injections. Most practitioners will mix lidocaine with hyaluronidase to make the procedure more comfortable.
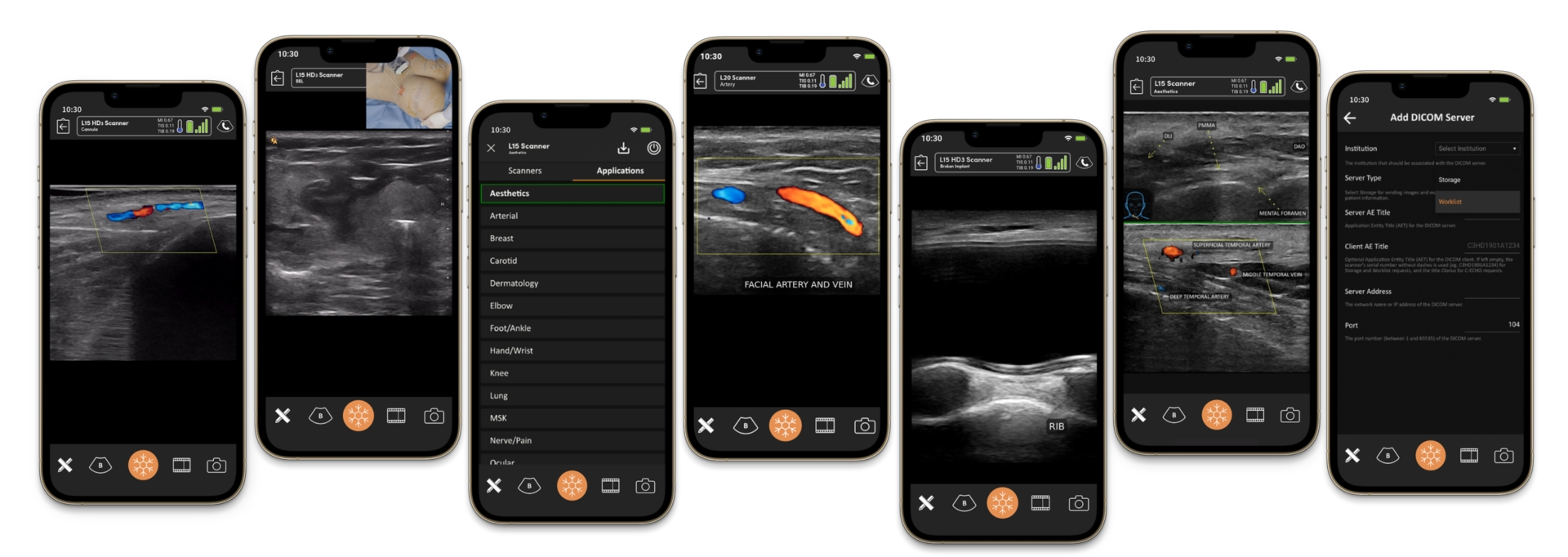
The Role of Ultrasound
High-frequency ultrasound is emerging as a valuable tool in managing filler complications. Ultrasound imaging allows practitioners to:
- Visualise the Filler: Confirm the presence and location of the filler, especially in cases of migration or nodule formation.
- Identify Vascular Occlusions: Detect disruptions in blood flow, aiding in the diagnosis of vascular occlusions.
- Guide Hyaluronidase Injections: Ensure accurate delivery of hyaluronidase to the target area, maximising its effectiveness and minimising the risk of damage to surrounding tissues.
- Monitor Dissolution: Assess the filler’s breakdown in real time, allowing for adjustments in dosage and technique as needed.
However, ultrasound is a complex skill that requires specialised training.
The Importance of Patient Education
In aesthetic practice, patient education is paramount.
- Realistic Expectations: Ensure patients have realistic expectations about the procedure and potential outcomes.
- Potential Risks and Complications: Discuss the potential risks and complications associated with dermal fillers, including the possibility of needing hyaluronidase.
- Informed Consent: Obtain informed consent before proceeding with any treatment, ensuring that the patient understands the procedure and its risks and benefits.
- Aftercare Instructions: Provide clear and detailed aftercare instructions to minimise the risk of complications and promote healing.
- Follow-Up: Schedule follow-up appointments to assess the results of the treatment and address any concerns or complications that may arise.
Limitations and Future Directions
While Hyal is a valuable tool, it’s important to acknowledge the limitations in the available research. The authors of the source document noted the limitations, as well as the variability in study designs and limited controlled data. The temporary contact between hyaluronidase and the HA filler on a plate may not account for the sustained enzymatic interaction that occurs in biological tissues. Moreover, the short experimental duration of 1 h contrasts with clinical evidence indicating that hyaluronidase’s effects can persist for up to 48 h post injection. Future studies should explore longer observation periods and more complex in vivo models to better understand hyaluronidase’s long-term effects. More high-quality, controlled studies are needed to establish standardised guidelines for hyaluronidase use.
Key Takeaway Messages
- Hyaluronidase is a crucial tool for managing HA filler complications.
- Different Hyal products have slightly different properties.
- Proper dosage and injection technique are critical for optimal results.
- Early recognition and prompt treatment of vascular occlusions are essential to prevent serious complications.
- Patient education and informed consent are paramount.
- Consider ultrasound guidance for more accurate results.
- The research and protocols are constantly evolving. Stay up-to-date.
The Bottom Line: Mastering Hyaluronidase for Safer Practice
Hyaluronidase is an indispensable asset for any aesthetic practitioner working with HA fillers. By understanding its properties, proper usage, and dosage guidelines, injectors can significantly improve patient safety and effectively manage complications. As the aesthetic field evolves, ongoing research and standardised protocols are essential to optimise Hyaluronidase use and ensure the best possible outcomes for our patients.
FAQ
How does hyaluronidase interact with different injectable fillers?
Hyaluronidase interacts differently with various hyaluronic acid (HA) dermal fillers, depending on several factors:
- HA concentration: Fillers with higher HA concentrations are generally more resistant to degradation by hyaluronidase.
- Crosslinking density: More extensively crosslinked fillers are harder for hyaluronidase to break down, as it has difficulty accessing its binding sites within the HA.
- Filler structure: Monophasic fillers tend to be less soluble in hyaluronidase compared to polyphasic fillers, as they have less surface area exposed to the enzyme.
- Particle size: Larger particle sizes contribute to increased durability and resistance to hyaluronidase degradation.
Studies have shown varying results regarding which specific fillers are most resistant to hyaluronidase:
- One study found Restylane dissipated most quickly, while Belotero was most resistant.
- A more recent study concluded that Belotero was the fastest to dissolve, while Juvederm Voluma and Restylane Lyft were the slowest.
The interaction between hyaluronidase and HA fillers is time- and dose-dependent. Different hyaluronidase products (e.g., Vitrase and Hylenex) appear to have similar effects, suggesting they may be used interchangeably.
It is essential to note that hyaluronidase may degrade the body’s natural hyaluronic acid (HA) in preference to injected HA fillers, which are specifically cross-linked to resist breakdown. Therefore, practitioners must carefully consider dosage and injection technique when using hyaluronidase to reverse filler complications.
What are the most common side effects associated with hyaluronidase use?
The most common side effects associated with hyaluronidase use are primarily local reactions at the injection site. These include:
- Pain, discomfort, or tenderness
- Swelling or oedema
- Redness or erythema
- Itching or pruritus
- Bruising or discolouration of the skin
Other common side effects reported include:
- Headache
- Fatigue
- Nausea
- Fever
These side effects are generally mild and transient, often resolving within a few hours after injection. It is essential to note that severe allergic reactions are rare, occurring in fewer than 0.1% of patients.
While these side effects are generally well-tolerated, patients should be monitored for signs of more serious reactions, particularly allergic ones, which may require immediate medical attention.
How common are allergic reactions to hyaluronidase?
Allergic reactions to hyaluronidase are relatively rare, but they can occur. The reported incidence of allergic reactions varies slightly across different studies and medical contexts:
- The overall incidence of allergic reactions to hyaluronidase is estimated to be between 0.05% to 0.69%.
- Urticaria and angioedema have been reported to occur at a low frequency of less than 0.1%.
- The estimated incidence of allergic reactions to hyaluronidase in ophthalmic surgery departments was approximately 0.1%.
- Some sources suggest that approximately one in two thousand people (0.05%) will have some kind or level of allergy to hyaluronidase.
It is essential to note that the risk of allergic reactions may increase with higher doses of hyaluronidase. For instance, when the dose exceeds 100,000 IU through intravenous injection, the likelihood of allergic reactions increases, and at doses of 200,000 IU, the occurrence of allergic complications can rise to 31.3%.
While most allergic reactions to hyaluronidase are local and relatively mild, systemic reactions can occur, especially with intravenous administration. Most of these reactions are immediate hypersensitivity reactions (Type I, IgE-mediated), but delayed hypersensitivity reactions (Type IV, T-cell-mediated) may also occur.
Despite the low incidence, healthcare providers should be aware of the possibility of allergic reactions when using hyaluronidase and monitor patients accordingly.
How effective are skin tests in predicting allergic reactions to hyaluronidase?
Skin tests for predicting allergic reactions to hyaluronidase have limited effectiveness and are not recommended as a routine screening tool:
- Skin tests are most useful for diagnosing Type I (IgE-mediated) hypersensitivity reactions when there is a clinical history suggestive of allergy.
- The British Society of Allergy and Clinical Immunology (BSACI) advises against using skin tests to screen for drug allergies in the absence of a compatible clinical history.
- While skin tests can help diagnose immediate hypersensitivity reactions, they may not predict delayed hypersensitivity reactions (Type IV, T-cell-mediated), which can occur even 24 hours after exposure.
- The reliability of skin tests for hyaluronidase allergy is questionable due to the lack of validated test concentrations.
- Some clinicians perform skin tests with 3 IU of hyaluronidase; however, this practice is often challenging to implement in general clinical settings.
- There is usually no clear link between a patient’s history of allergies and their response to hyaluronidase, further complicating the predictive value of skin tests.
In conclusion, while skin tests can be helpful in specific cases with a suggestive clinical history, they are not reliable as a routine screening tool for predicting allergic reactions to hyaluronidase. Clinicians should be aware of the limitations of these tests and interpret results cautiously within the appropriate clinical context.
How reliable are intradermal tests compared to skin prick tests for hyaluronidase?
Intradermal tests are generally more sensitive than skin prick tests for detecting allergic reactions to hyaluronidase, but they also carry a higher risk of adverse reactions:
- The sensitivity of intradermal tests (73%) is significantly higher than skin prick tests (49%) for detecting adverse cutaneous drug reactions.
- Intradermal testing is considered more sensitive but less specific than skin prick testing for detecting immediate hypersensitivity reactions1.
- However, intradermal testing carries a higher risk of systemic reactions, including anaphylaxis, compared to skin prick testing1. There have been reports of deaths from intradermal testing, while only one death has been reported from skin prick testing.
- For hyaluronidase specifically, intradermal testing is not considered reliable due to the lack of validated test concentrations3. Performing intradermal tests with unvalidated concentrations in high-risk patients (e.g., those with a history of bee/wasp sting allergies) could potentially result in anaphylaxis.
- Some studies have found intradermal tests to be more sensitive than skin prick tests for hyaluronidase. For example, one case report showed positive intradermal test results for hyaluronidase at both 1:1 and 1:10 dilutions, while skin prick tests were negative.
In conclusion, while intradermal tests may be more sensitive, they are not necessarily more reliable for hyaluronidase testing due to the higher risks involved and lack of validated protocols. Skin prick tests are generally considered safer and more appropriate for initial screening, with intradermal testing reserved for specialist settings when necessary.
Can hyaluronidase cause long-term skin damage
Based on the available evidence, there is no conclusive scientific proof that hyaluronidase causes long-term skin damage when used properly for dissolving dermal fillers. However, some patients have reported experiencing prolonged adverse effects after hyaluronidase treatment.
Most medical literature suggests that hyaluronidase is safe and effective when used appropriately. Studies have shown that while hyaluronidase can temporarily degrade natural hyaluronic acid in the skin, this effect is short-lived as the body rapidly replaces it. Long-term adverse reactions are considered rare, with most being related to foreign body-type inflammatory responses rather than permanent tissue damage.
However, there are numerous anecdotal reports from patients claiming long-lasting adverse effects after hyaluronidase treatment. These include facial volume loss, skin laxity, nerve damage, and accelerated ageing. It is essential to note that these reports are not scientifically verified and may be influenced by factors beyond the hyaluronidase treatment.
Some experts acknowledge that hyaluronidase may cause real physical problems in a minority of patients6. The mechanisms behind these potential long-term effects are not fully understood and require further research.
It is crucial to remember that the use of hyaluronidase for dissolving dermal fillers is considered off-label. Patients should be fully informed of potential risks and benefits before undergoing treatment. Proper technique, appropriate dosing, and careful patient selection are essential to minimise the risk of adverse effects.
In conclusion, while the scientific consensus suggests that hyaluronidase is generally safe, the possibility of long-term effects in some individuals cannot be entirely ruled out. More research is needed to fully understand the long-term impacts of hyaluronidase on skin health.